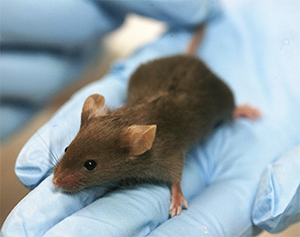
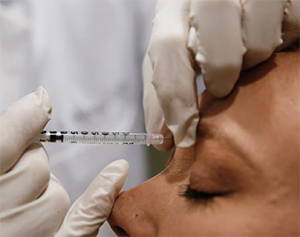
Dogs and cats were among more than 106,000 live animals used for scientific research and testing last year, representing an increase of around 15 per cent compared to 2022. There was also a significant rise in the number of experiments involving “severe” suffering for the test animals. These rose by almost 50 per cent to 19,816 during 2023.
This increase has been attributed to the expansion of testing for Botox-type products, most of which relate to cosmetics. Around 68,500 animals were used in these tests last year, which are fatal in the vast majority of cases. Mice were the most commonly used animal in scientific research and testing in Ireland last year, accounting for 81 per cent of the 106,639 animals subjected to procedures.
A total of 14,105 of the animals were used for “basic research”, according to the HPRA, while 17,527 were used for translational and applied research. Regulatory use and routine production accounted for 75,109 of the animal uses, which included the testing of Botox-type products.
The Irish Anti-Vivisection Society, which campaigns against the use of animals in scientific research, described the increase in animal suffering outlined in the latest report as “appalling”.