Botulinum toxin (Bt) is a neurotoxin which induces muscle paralysis by blocking neurotransmitters. Bt is used for treating medical conditions (such as migraines and lazy eye), and in cosmetic procedures to reduce the appearance of wrinkles.
The manufacture of Bt products is approved for medical purposes – and the use of animals in testing procedures is authorised for that reason. However, huge numbers of practitioners use them “off-label” for aesthetic treatments. “Off-label use” is known to be widespread, but it is not known what percentage is used for cosmetic versus medical applications. Indeed, there has been a documented surge in demand for Bt-based products within the cosmetic industry. In 2022, the International Society of Aesthetic Plastic Surgery estimated over 9 million “Botox” treatments were administered, a global increase of 26.1% compared to 7 million in 2021.
As Bt is a biological product, the potency of each batch needs to be tested and assured before it can be used in humans. The standard method, an LD50 assay using mice, determines the lethal dose that kills 50% of test animals. The increasing demand of Bt products has involved large numbers of animals undergoing ‘severe’ procedures.
In recent years, there has been substantial progress towards the replacement of Bt testing in animals. The manufacturer of Botox®, Allergan, was the first to develop its own cell-based potency assay in 2011, aimed at replacing the use of animals in batch potency testing for Botox. Allergan is based in Ireland, and a review of the available statistics from 2013-2022 reveals a marked decrease in the number of animals used in regulatory testing and undergoing severe procedures.
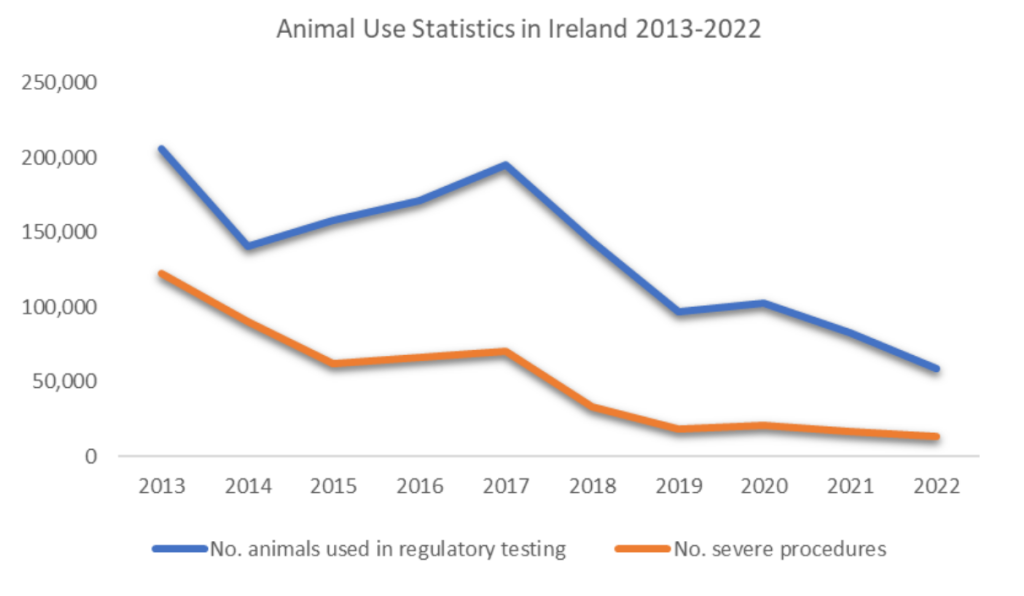
Between 2013 and 2022, there was an 89% decrease in the number of severe procedures in Ireland. The statistical reports do not provide enough detail to exclusively credit this decline to the replacement of batch potency testing of Bt. However, it is certainly feasible that the approval and use of the cell-based potency assay has played a significant role. If this is the case, transitioning from traditional animal testing methods to in-vitro alternatives substantially reduced severe suffering.
Unfortunately, the LD50 test continues to be used for batch potency testing of other versions of ‘Botox’ type products in Ireland and globally. Because each manufacturer produces Bt slightly differently, an alternative to the animal test needs to be validated for every company, and regulators in all jurisdictions where the product will be used need to accept these. Unfortunately, more animals will experience severe suffering until this is achieved. Also, manufacturers who are successfully using a cell-based alternative for batch potency tests may still use animals for other parts of the production process. The significant animal suffering still involved in the production of these products highlights the importance, and urgency, of enabling more rapid transitions to non-animal technologies and approaches.
